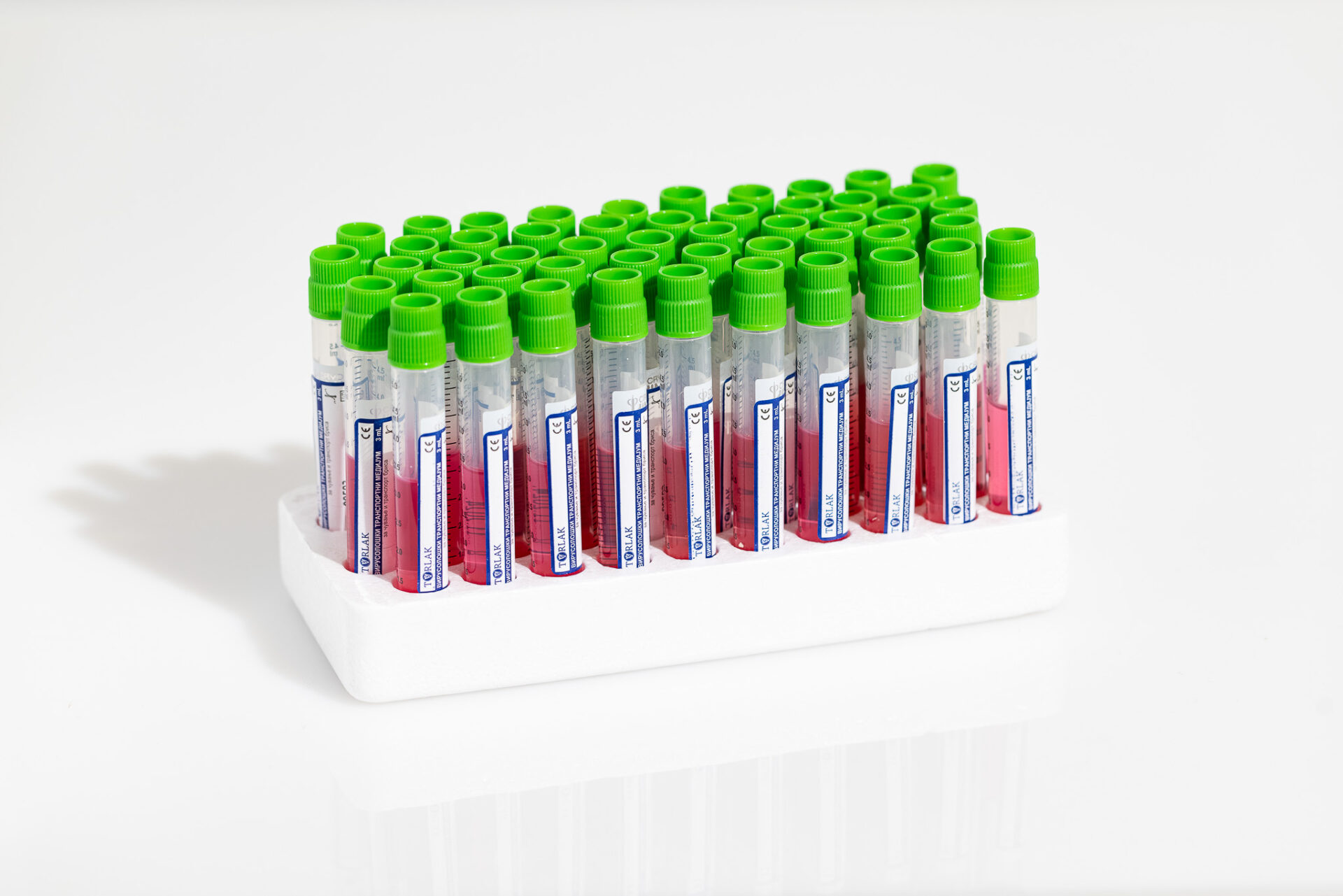
Virological Transport Medium (VTM) is a product designed for transporting samples collected by swab for virological analysis.
The formulation of Virological Transport Medium is such that it allows the preservation of virus viability without the possibility of its replication.
The product contains antibiotics and fungicides that cover a broad spectrum of activity. This prevents the growth of bacteria and fungi in the medium.
The medium is suitable for transporting the following viruses: Parainfluenza, Enterovirus, Adenovirus, Herpes simplex virus, Influenza virus type A and B, Respiratory syncytial virus (RSV), Varicella Zoster virus, Rhinovirus, SARS-CoV-2 virus, Cytomegalovirus (HCMV), Metapneumovirus, Monkeypox virus, and other viruses.
The medium is suitable for transporting bacteria of the genus Mycoplasma.
The medium is suitable for both molecular diagnostics based on RT-PCR and for virus isolation and cultivation on cell lines (tissue culture).
The product is formulated according to the guidelines of CDC, WHO, ATCC, and FDA.
For the production of VTM, the Institute of Virology, Vaccines, and Sera “Torlak” holds the ISO13485:2016 certificate.